This week, the Food and Drug Administration informed multiple drug companies that they must include a boxed warning, the agency’s most stringent safety label, in the ordering information for CAR-T therapy, a form of cancer treatment.
According to the warning, the treatment may potentially increase the likelihood of developing cancer.
A spokesperson from the FDA, Carly Kempler, stated that despite the warnings issued for these products, their potential risks are outweighed by their overall benefits.
According to Kempler, the government made the decision to change the labels after receiving reports of rare blood cancers in individuals who had previously undergone CAR-T treatment.
According to her, the government has received 25 reports of blood cancers in CAR-T patients as of Monday.
In a recent statement, Bruce Levine, a professor of cancer gene therapy at the University of Pennsylvania, highlighted the findings of two abstracts published in the journal Blood.
These abstracts raised concerns about a potential cancer risk associated with CAR-T therapy. According to him, this probably compelled the FDA to take action.
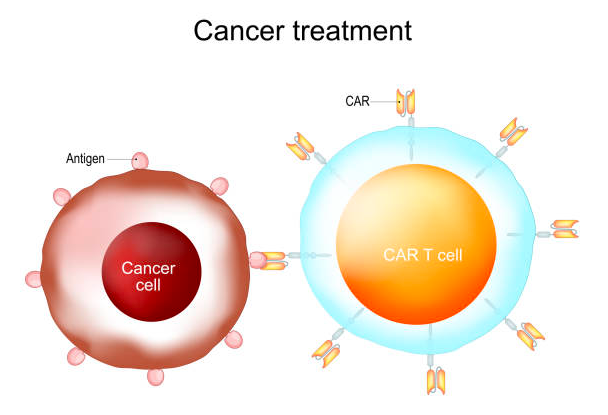
CAR-T treatment is a groundbreaking therapy that effectively targets certain blood cancers by utilizing the patient’s own immune cells.
There are various types of cancer, such as leukemia, multiple myeloma, and lymphoma. The process involves extracting T cells, which are immune cells, from the patient.
These cells are then genetically modified in a laboratory to enable them to target cancer cells. Finally, the modified cells are reintroduced into the patient’s body.
According to experts, it is highly effective in challenging treatment scenarios. In 2022, medical professionals who had utilized CAR-T therapy to address leukemia in two individuals a decade ago confidently declared that the treatment had effectively resulted in their complete recovery.
Dr. Matthew Frigault, who oversees the Cellular Immunotherapy Program at Massachusetts General Hospital in Boston, expressed that this discovery has significant implications for the treatment of lymphoma and other diseases.
In 2017, the FDA granted approval to Novartis’ Kymriah, making it the first CAR-T treatment to receive such recognition. And since then, five additional individuals have been terminated.
Abecma and Breyanzi were developed by Bristol Myers Squibb, while Yescarta was created by Gilead Sciences’ Kite Pharma.
Carvykti was produced by Johnson & Johnson, and Kymriah was developed by Novartis. The companies that manufactured the drugs received letters from the FDA, instructing them to submit proposed label changes within 30 days.
These changes would include a warning about the potential increase in the risk of rare blood cancers associated with CAR-T therapy. Kite Pharma did not receive any correspondence regarding Tecartus, the sixth CAR-T drug.
If the drug companies disagree, they have the option to send a response explaining their reasons for why the rules should remain unchanged.
According to Novartis, there is currently no substantial evidence to support a connection between their treatment and cancer, despite being administered to over 10,000 individuals.
A business representative mentioned their willingness to collaborate with the FDA in order to make suitable modifications to the label.
Johnson & Johnson and Gilead Sciences have expressed their commitment to collaborating with the FDA in order to implement necessary modifications to their labels.
Bristol Myers Squibb stated that they are considering their “next steps” following the FDA’s warning, despite not having observed any instances of cancer associated with their treatment.
Understanding Cancer Risks of CAR-T Treatment
However, the exact mechanism by which CAR-T could potentially lead to the development of cancer remains uncertain.
CAR-T therapies are relatively recent developments: According to Frigault, the FDA has instructed the manufacturers of these products to conduct follow-up studies every 15 years to assess the likelihood of cancer recurrence after treatment.
(Secondary cancers are a potential occurrence following treatment.)
He mentioned that the FDA has not stated that every case they have reported definitively proves that CAR-T has caused this. On the contrary, there are suggestions of a possible connection.
According to Dr. Hemant Murthy of the Mayo Clinic in Jacksonville, Florida, the risk of CAR-T therapy leading to cancer is minimal.
According to the FDA, over 27,000 doses of CAR-T treatment have been administered in the United States.
According to Dr. Saad Usmani, a myeloma doctor and cell therapist at Memorial Sloan Kettering, the label change should align with current medical practices.
This involves discussing with patients the potential risk of developing another cancer following treatment for their initial cancer.
According to Usmani, other cancer treatments such as chemotherapy and radiation can also raise the risk of developing a second cancer.
“The change is probably due to recent reports, although it is a rare occurrence,” he mentioned.
Dr. Marcela Maus, an esteemed medical professional from Harvard Medical School and Massachusetts General Hospital, expressed that while doctors may exercise more caution, it is unlikely to significantly alter their approach to their work.
Read also: Study Reveals HPV Vaccine’s Success in Eradicating Cervical Cancer Among Young Women